What if you're forced to substitute biologic to biosimilar?

Consider the situation where you have a serious chronic arthritis. It took time to diagnose, then time to work through a number of medication combinations before you & your rheumatologist finally found the right biological DMARD for you.
Let's say it's working well and you're happy with the results.
Then, the funding body, be that an insurance company or the government health body in charge, decides that you no longer need to be on that particular brand. Instead, they make the choice for you or leave it to the pharmacy to swap you to another drug.
That would be OK if the other drug was IDENTICAL.
The problem is that with biologic DMARD therapy, this is not the case. These are large, complex, molecular compounds that are synthesised in living cell lines.
We aren't talking about Paracetamol, a simple molecule that you can make generics from easily.
There are not any biologic DMARD generics.
There are a group of medications coming called biosimilars. These are agents which are designed to be similar but it's technically impossible to make them identical.
Now, there is great hope for these biosimilar compounds. Choice is important and more options are great (because they are different from what we already have). In addition, they will be cheaper than the originator compounds and should lead to reductions in price of the biological DMARDs in time.
But, the important thing is choice.
Most people would not like to be forced to swap from a medication they tolerate well and/or have a good result from to a different, yet similar, medication which they have never tried. Disease control can be lost, side effects may occur, drug resistance could possibly occur.
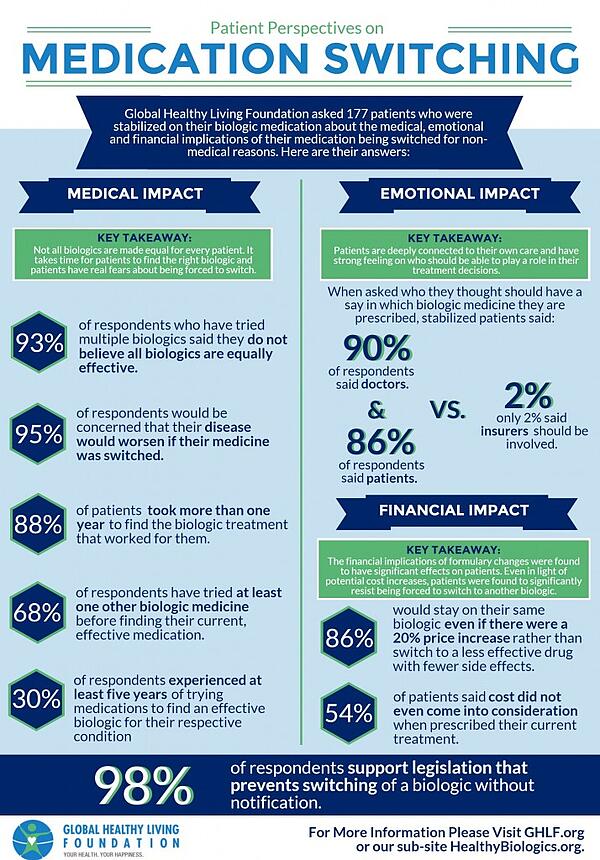
Seth Ginsberg, from CreakyJoints shared with me the following survey results.
A regular contributor to this blog, Naomi Creek, who is the coordinator for CreakyJoints Australia is fighting moves in Australia to allow this forced substitution.
What are your thoughts?