Can we use TNF inhibitors early then take them away?

By Dr Irwin Lim, Rheumatologist
I think that increasingly rheumatologists would like to be able to use TNF inhibitor medications much earlier in the course of rheumatoid arthritis (RA).
These drugs are effective in RA and the hope would be that earlier use leads to higher rates of remission, lower rates of joint damage, and ultimately better long term outcomes.
There are some patients who come into my room with quite severe arthritis at presentation and the goal is clearly to get disease control as soon as possible.
Understandably, early use of these agents in many countries, including Australia, is not government funded. This is due to their high cost and the argument is that we, the taxpayers, just can't afford it.
What may change this?
Well, we have been waiting for clinical trials to give us insights into strategic use of these medications.
In the November 6th issue of the New England Journal of Medicine, such a trial has been published (read the abstract here).
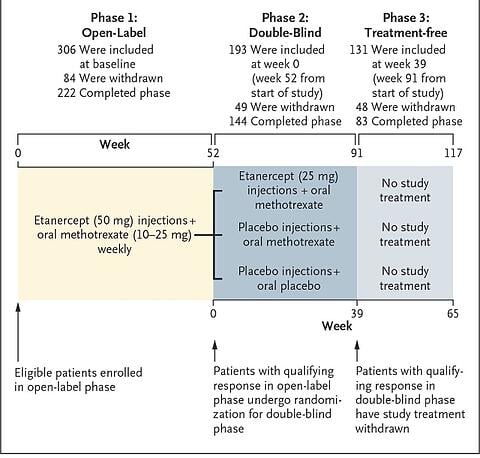
This 3-phase trial tried to test the value of using Etanercept/Methotrexate in combination in patients with early RA ("early" in this case, meaning those with onset of symptoms within 12 months of enrolment) and then taking away the medication.
306 patients from multiple different centres in Europe and Asia were enrolled. The design was as follows:
- Phase 1: treat early RA with weekly 50mg Etanercept plus weekly oral Methotrexate (MTX) for a total of 52 weeks.
- Phase 2: those who achieved low disease activity at week 39 and remission at week 52 were then randomised to one of 3 groups. These 3 groups were:
- half-dose Etanercept at 25mg weekly plus MTX, or
- MTX alone, or
- Placebo treatment only.
- Phase 3: At week 39 after randomisation into the phase 2 groups, all those who continued to have a good response were taken off all medications and followed up to week 65 (post-randomisation).
A complicated trial. Trying to answer some important questions.
I'm sure it will be analysed and debated by the experts in detail but the initial take-home messages are:
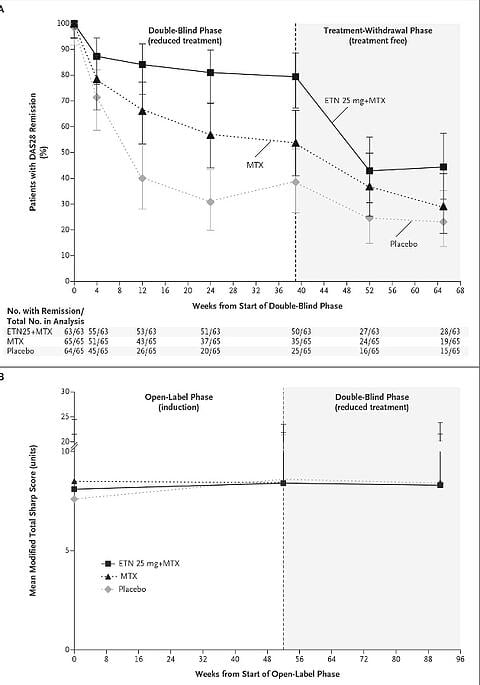
- A high percentage of patients in phase 1 reached remission (70%) according to DAS28 criteria.
- In the step-down therapy phase, phase 2,
- 40 (63%) of 63 patients in the half-dose etanercept plus MTX group vs 26 (40%) of 65 patients in the MTX-only group vs 15 (23%) of 65 patients in the placebo group achieved DAS28 remission at weeks 24 and 39.
- Of the patients who continued to have low disease activity who entered phase 3, and who had all their active medication stopped,
- 44% of the phase 2 combination group continued to be in DAS28 remission at week 65 after randomisation vs 29% of the MTX-only group, vs 23% of the placebo group.
- There were not any significant changes in the progression of damage on Xrays
So, this trial seems to provide evidence that once we achieve remission in an early group of rheumatoid patients using combination Etanercept/MTX therapy, we are then able to reduce and even withdraw the TNF inhibitor. In some, we can even withdraw MTX.
We still don't know how to pick which patients will be able to have their medications stopped without the disease flaring.
So, in practice, rheumatologists will just taper therapy cautiously, monitor patients carefully, and restart treatment upon flare.
Dr Irwin Lim is a rheumatologist and a director of BJC Health. You should follow him on twitter here.BJC Health’s vision is to create best care for people with arthritis. Contact us.
Subscribe to Dr Lim's Blog
This blog focuses on arthritis, healthcare in general, and Connected Care.
Enter your name and email address to subscribe to this blog and receive notifications of new posts by email.